(a)(i) Define the term standard electrode potential.
(ii) State three factors that affect the discharge of ions during electrolysis.
(iii) State two functions of a salt bridge in an electrochemical cell.
(b) Describe briefly what happens when a solution of copper (II) tetraoxosulphate (VI) is electrolyzed using copper electrodes.
c) Calculate the mass of copper deposited at the cathode when a current of 0.2A is passed through a solution of copper (II) tetraoxosulphate (VI) for 35 minutes using copper electrodes. [H = 1.00, O = 16.0, S = 32.0, Cu = 64.0, IF = 96,500C]
(d)(i) State three characteristics of a catalyst.
ii) Name one manufacturing process in which each of the following metals is used as catalyst: I. iron; II. nickel; Ill. platinum.
c-NA
(a)(i) Define the term hygroscopic.
(ii) Give two difference: between a physical change and a chemical change.
(iii) Using the kinetic theory of gases, explain briefly the Charles' law.
(b)(i) Arrange the following compounds in order of increasing boiling points: CS\(_2\); CO\(_2\); NaH. Give reasons for your answer.
(ii) Write a balanced chemical equation to illustrate the reaction of chlorine gas with cold dilute sodium hydroxide.
(c) In a certain reaction, 15.0 g of impure magnesium sample reacted with excess hydrochloric acid liberating 8.6 dm\(^2\) of hydrogen gas at s.t.p.
(i) Write a balanced equation for the reaction.
(ii) Calculate the: I. mass of pure magnesium in the sample; I. percentage purity of the magnesium sample; III. number of ions produced in the reaction. [Mg = 24.0; volume at s.t.p. 22.4 dm\(^{-3}\), Avagadro's constant = 6.02 x 10\(^{23}\)mol\(^{-1}\)]
(a)(i) Explain briefly the term chemical industry.
(ii) State three factors that should be considered in siting a chemical industry.
(b)(i) Describe briefly twin is extracted from its ore.
(ii) Give two uses of tin.
(c)(i) Name the constituents of cement.
(ii) How does mortar set?
(d)(i) Explain briefly the term pollution.
(ii) Give two examples of air pollutants.
(e) Consider the following reversible reaction which occurred at the temperature of 298K:
N\(_{2(g)}\) + 3H\(_{2(g)}\) \(\rightleftharpoons\) 2NH\(_{3(g)}\); \(\bigtriangleup\)H = —92.37kJ
(a)(i) List two characteristics of homologous series.
(ii) Consider the compound represented by the following formula: CH\(_3\)(CH\(_2\))\(_2\)CH\(_3\). I. Which homologous series does the compound belong? II. Write the structures of three possible isomers of the coumpound. III Name the three possible isomers in (a)(ii)II.
(b) Write the structure of the major product formed in each of the following reactions: (i) ethanol with excess acidified potassium tetraoxomanganate (VII);
(ii) excess ethane with chlorine in the presence of sunlight;
(iii) ethanol with propanoic acid in the presence of few drops of concentrated tetraoxosulphate(VI) acid.
(c) Name the major product in each reaction in (b).
(d) Consider the following organic compounds:
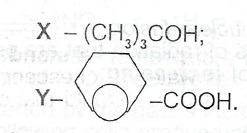
(i) Give the IUPAC name of each compound.
(ii) State a chemical test for the functional group in each compound.
(e) An organic compound with relative molecular mass 136 contains 70.57% carbon, 5.90% hydrogen and 23.53% oxygen. Determine its: (i) empopirical formula; (ii) molecular formula. [H= 1.00, C= 12.0, H = 16.0]
(a) (i) What is the structure of the atom as proposed by Rutherford?
(ii) Distinguish between the atomic number and the mass number of an element.
(iii) Explain briefly why the relative atomic mass of chlorine is not a whole number.
(b) (i) What is meant by first ionization energy?
(ii) List three properties of electrovalent compounds.
(iii) Consider the following pairs of elements: I. \(_9\)F and \(_{17}\)Cl; \(_{12}\)Mg and \(_{20}\)Ca. Explain briefly why the elements in each pair have similar chemical properties.
(c) Explain briefly the following terms using an appropriate example in each case.
(i) homologous series; (ii) heterolytic fission.
(d) State the indicator(s) which could be used to determine the end-point of the following titrations:
(i) dilute hydrochloric acid against sodium hydroxide solution;
(ii) dilute hydrochloric acid against ammonium hydroxide solution;
(iii) ethanoic acid against sodium hydroxide solution.
(e) A solid chloride E which sublimed on heating reacted with an alkali F to give a choking gas G. G turned moist red litmus paper blue. identify E, F and G.