(a) Describe briefly a suitable procedure for preparing a pure sample of MgSO\(_4\) starting from MgO.
(b)(i) Mention two sources of water pollution.
(ii) Explain why the sample of air collected in the process of boiling water is richer in oxygen than atmospheric air
(iii) Mention one substance used as coagulant in water treatment plants.
(c)(i) State two physical porperties of chlorine.
(ii) Write an equation to show how chlorine reacts with iron
(iii) Why is Chlorine preferred to sulphur (IV) oxide in the bleaching of cotton
(d) Bleaching powder reacts with dilute HCl according to the reaction below;
CaOCl\(_{2(s)}\) + 2HCI\(_{(aq)}\) -> CaCl\(_{2(aq)}\) + H\(_2\)O\(_{(l)}\) + Cl\(_{2(g)}\)
Calculate the mass of bleaching powder that will produce 400cm\(^3\) of chlorine at 25\(^o\)C and a pressure of 1.20 x 10\(^5\) NM\(^{-2}\). [O = 16.0; Cl = 35.5; Ca = 40.0;1 mole of gas occupies 22.4 dm\(^3\) at s.t.p; standard pressure = 1.01 x 10\(^6\) Nm\(^{-2}\)]
(a)(i) List three properties of elements which increase generally across a period in the Periodic Table,
(ii) Give two differences between a chemical reaction and a nuclear reaction.
(b) Use the information provided in the table below to answer Questions (i) - (vii).
|
Atom of Element |
P | Q | R | S | T |
|
Mass Number |
16 | 40 | 35 | 18 | 20 |
|
Atomic Number |
8 | 20 | 17 | 8 | 10 |
Which of the atoms in the table above:
(i) are isotopes of the same element?;
(ii) contains 18 neutrons?;
(iii) is chemically unreactive?;
(iv) readily forms an ion with two positive charges?
(v) attain an octet structure by accepting one electron?;
(vi) forms ionic bond with R?;
(vii) belongs to the s-block in the Periodic Table?
(c) Describe in outline how each of the following conversions can be carried out in the laboratory. Write appropriate equations for the reactions involved in each case
(i) CuCO\(_3\) to Cu
(ii) MgO to MgSO\(_4\).
a)(i) State Graham's law of diffusion.
(ii) Calculate the vapour density of a triatomic gas X if its relative: atomic mass is 16.
(iii) Equal volumes of gases Y and Z are maintained at the same temperature and pressure. If the mass of a molecule of Y is twice that of Z state and explain which of the molecules has the, greater average velocity.
(b) The graph below is the ratio curve for the following reaction carried out in an open vessel.
MgCO\(_{3(s)}\) + 2HCI\(_{(aq)}\) \(\to\) MgCl\(_{2(aq)}\) + CO\(_{2(g)}\) + H\(_2\)\(_{(l)}\).

(i) For how long did reaction occur?
(ii) Why was there a loss in mass?
(iii) State whether reaction rate was fastest at the beginning, the middle or towards the end of the reaction. Give reason for our answer.
(iv) List three reaction conditions that can affect the slope of the curve
(c) Consider the following reaction at equilibrium: PCI\(_{5(g)}\) \(\rightleftharpoons\) PCI\(_{3(g)}\)); \(\Delta\)H = +95 kJmol\(^{-}\)
(i) Write an expression for the equilibrium constant K.
(ii) Predict the effect of the following on the equilibrium position.
I. Increased pressure
II. Increased temperature
III. Removal of chlorine Sketch an energy profile diagram for the forward reaction.
(a)(i) List two properties of iron that are characteristic of transition metals
(ii) Using equations only, show the processes involved in the extraction of iron and the removal of impurities in the blast furnace.
(iii) The following reaction occurs when a piece of iron is exposed to moist air for some days:
4Fe\(_{(s)}\) + 3O\(_{2(g)}\) + xH\(_2\)O\(_{(l)}\) —> 2Fe\(_2\)O\(_3\)\(_{(s)}\) State three methods by which this reaction can be prevented
(iv) What is the oxidation number of iiron in the product in (iii) above?
(b)(i) Arrange the following metals in the order of increasing reactivity. Hence, state which of them is/are extracted by electrolysis Au, Zn, Mg, Na, Sn, Ca
(ii) Why is zinc said to be amphoteric?
(c)(i) Define oxidation in term of electron transfer
(ii) Determine how many moles of electrons are transferred when 4825 coulombs of electricity are passed through an electrolytic cell. [1F = 96500C]
(iii) Calculate the number of copper (II) ions that will be discharged by 0.250F. [Avogadro constant = 5.02 x 10\(^{23}\)
(a) What is meant by each of the following terms?:
(i) Esterification
(ii) Saponification
(b)(i) Give the general moluecular 1 formula of alkynes
(ii) Write the molecular formula and empirical formula of ethylethanoate.
(iii) Draw the structure of 1, 1, 2, 2-tetrabromoethane
(iv) Write an equation for the reaction of ethanol with sodium
(c) Consider the following reaction schemes:
I II
Petroleum ---> Petroleum Fractions. Higher Petroleum Fractions ---> Petrol + X
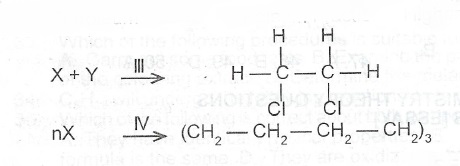
(i) State type of process/reaction involved in each of the stages labelled I to IV.
(ii) Identify X and Y
(iii) Give the IUPAC name of the product obtained in stage III.
(iv) What are the reaction conditions for stage IV?
(d) Explain why palm wine: (i) froths or foams (ii) tastes sour after some days.