(a) Giving different examples, mention one metal in each case which produces hydrogen on reacting with
(i) dilute mineral acid
(ii) cold water;
(iii) steam;
(iv) hot, concentrated alkali.
(b) In an experiment, excess 0.50 mol dm\(^{-3}\) HCI was added to 1Og of granulated zinc in a beaker. Other conditions remaining constant, state how the reaction rate would be affected in each case, if the experiment was repeated using:
(i) 1.0 mol dm\(^{-3}\) HCI;
(ii) 8.0g of granulated zinc;
(iii) 10g of zinc dust;
(iv) a higher volume of 0.50 mol dm HCI;
(v) a reaction vessel dipped in crushed ice;
(vi) equal volumes of water and 0.50 mol dm\(^3\) HCI.
(c) Aluminium is extracted from its ore by electrolysis.
(i) Name the ore from which the metal is extracted.
(ii) State the role of molten cryolite in the extraction.
(iii) Describe in outline how the ore is purified before electrolysis
(iv) Calculate the current in amperes required to produce 18.0g of aluminium in 1.50 hours. [Al = 27.0; F = 96500C]
(d) Give the reason why
(i) aluminium, which is a reactive metal, is resistant to corrosion.
(ii) metals are generally good reducing agents.
(a) What term is used to describe each of the following processes?
(i) Alkaline hydrolysis of fats and oils;
(ii) The conversion of glucose into ethanol by enzymatic action;
(iii) Thermal decomposition of higher petroleum fractions into lower molecular mass hydrocarbons in the presence of catalyst.
(b)(i) Write the structure and IUPAC name for one alkanoic acid with the molecular formula C\(_4\)H\(_8\)0\(_2\).
(ii) Arrange the following compounds in order of increasing boiling point: Butane; Butanoic acid; Methylpropane.
(iii) Give an explanation for your answer in (b)(ii).
(c)(i) Ethanol was used for preparing a gas X which decolorized bromine water. Identify X and describe briefly its laboratory preparation.
(ii) Write an equation to show how ethanol reacts with sodium
(iii) Give the reagent and reaction conditions for the conversion of ethanol into C\(_2\)H\(_5\)COOC\(_2\)H\(_5\).
(d) State the type of recction involved in each of the conversions indicated below:
(i)C\(_6\)H\(_6\)C\(_6\)H\(_5\)CH\(_3\)
(ii) nC\(_2\)H\(_4\) \(\to\) (CH\(_2\) - CH\(_2\)),
(iii) CH\(_3\)CH\(_2\)CH(OH)CH\(_3\) -> CH\(_3\)CH\(_2\)CCH\(_3\)
(iv) (C\(_6\)H\(_{10}\)O\(_5\)) -> C\(_6\)H\(_{12}\)O\(_6\).
(iv)
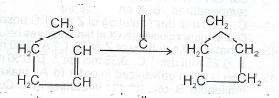
(a) Explain in terms of the kinetic theory why a tyre should not be overinflated.
(b)The following results were obtained at room temperature in an experiment to verify one of the gas laws using a glass syringe:
|
Pressure (P) of air in syringe (atm) |
Volume (V) of air in syringe (cm\(^3\) | \(\frac{I}{V}\) |
| 0.100 | 10.00 | 0.100 |
|
0.125 |
8.00 | 0.125 |
|
0.150 |
6.60 | 0.150 |
|
0.175 |
5.60 | 0.179 |
|
0.200 |
4.80 | 0.208 |
| 0.225 | 4.40 | 0.227 |
(i) Plot a graph of P against \(\frac{1}{v}\), using 1 cm to represent 0.01 atm on the vertical axis and 1cm to represent 0.02 unit on the horizontal axis.
(ii) Which of the gas laws is in agreement with the results?
(c) The flow chart below represents the stages involved in the manufacture of H\(_2\)SO\(_4\).
+x +Conc. H\(_2\)SO\(_4\) +H\(_2\)O
S + O\(_2\) \(\to\) SO\(_2\) \(\to\) SO\(_3\) \(\to\) Y \(\to\) Conc H\(_2\)SO\(_4\)
stage I stage II stage III stage IV
(i) Name the process represented by the chart.
(ii) Identify reactant X and product Y.
(iii) What are the operating temperature and pressure at stage II?
(iv) Mention the stage which requires a catalyst and state the catalyst used.
(v) Give the reason why the SO\(_3\) produced in stage II is not dissolved directly in water to form the acid
(d) When K\(_4\)Cr\(_2\)C\(_7\) dissolves in water, the following equilibrium is established:
Cr\(_2\)O\(^{2-}_{7(aq)}\) + H\(_2\)O\(_{(l)}\) \(\to\) 2CrO\(^{2-}_{4(aq)}\) + 2H\(_{aq}\)
(i) State the colour observed on adding a few drops of dilute H\(_2\)SO\(_4\) to the system.
(ii) Explain your answer in (d)(1).
(iii) What principle is applicable to this explanation?
(a)(i) State three methods of preparing salts, giving one example in each case of a salt so prepared.
(ii) What type of salt is each of the following? NaH\(_2\)PO\(_4\); (CH\(_3\)COO)\(_2\)Pb; KAI(SO\(_4\))\(_2\). 12H\(_2\)O.
(b)(i) Write an equation for the reaction between dilute HCI and a solution of AgNO\(_3\).
(ii) Explain why NaNO\(_3\) is preferred to AgNO\(_3\) in the preparation of oxygen by thermal decomposition of trioxonitrate (V) salts.
(iii) When silver wire was dipped into an aqueous solution of CuSO\(_4\), the wire remained intact but when the wire was replaced with zinc rod, the rod decreased in size. Give an explanation for this observation.
(c) When a sample of a crystalline salt X was exposed to air, there was a loss in mass.
(i) What phenomenon was exhibited by X?
(ii) Suggest two substances which X could be.
(iii) On heating 5.00 g of a fresh sample of X to constant mass, 1.80g was lost in the form of water vapour. Calculate the number of molecules of water of crystallization in one molecule of X. [H = 1.00; O = 16.00; Anhydrous form of X = 160 g mol\(^{-1}\)
a)(i) Give two uses of ammonia.
(ii) Name the process by which ammoniacal liquor can be obtained from coal and list two other products of the reaction
(iii) What type of reaction is involved in the conversion of ammoniacal liquor to (NH\(_4\))\(_2\)SO\(_4\) by dilute H\(_2\)SO\(_4\)?
(iv) Sketch and label an energy profile diagram to show the effect of presence of Pt/Rh on the reaction represented by the following equation: 4NH\(_3\) + 5O\(_2\) \(\to\) 6H\(_2\)O + 4NO; \(\Delta\)H = —907 kJmol\(^1\)
(b) Rock salt is an impure form of sodium chloride.
(i) Outline a suitable procedure for preparing a pure sample of sodium chloride from rock salt.
(ii) State two methods that can be used to prepare chlorine from rock salt. Write an appropriate equation in each case.
(c) Lead pigments were used in a water colour painting which turned black after prolonged exposure to an air pollutant. The original colour was restored by using H\(_2\)O\(_2\) which converted the black substance to a simple, white lead (II) salt.
(i) Which pollutant turned the painting black?
(ii) Write the formula of the black substance
(iii) What is the white salt?
(iv) State the role of H\(_2\)O in the restoration process.