a) Define the following in term:. of electron transfer: (i) oxidation; (ii) reduction.
(b)(i) Determina the oxidation stale of phosphorus in each of the following structures: I. POCI\(_3\) II. PH\(_3\).
(ii) State with reasons whether the following compounds will form acidic, neutral or basic aqueous solutions: I. NaNO\(_3\) II. Na\(_2\)H\(_4\)CI; Ill. Na\(_2\)CO\(_3\).
(c) Consider the set-up
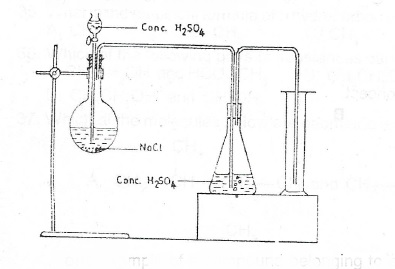
(i) What is the gas produced in the experiment illustrated by the set-up above?
(ii) Name the method of collection of gas
(iii) Give a reason for your answer in (c)(ii) above
(iv) State the function of the concentrated H\(_2\)SO\(_4\) in the conical flask
(v) Give of collection of the gas one I. physical property; II. chemical property of the gas
(vi) State one chemical test to identify the gas.
(d) A 4.3 g hydrated sodium tetraoxosulphate (VI) (Na\(_2\)SO\(_4\).xH\(_2\)O) was heated to remove the water of crystallization. The remaining anhydrous salt had a mass of 2.12 g. Calculate the value of x in the t I hydrated salt. [H = 1; O = 16; Na = 23; S = 32 ]
(a)(i) What is 2 homologous series?
(ii) Give two homologous series present in petroleum
(iii) Give one example of a compound belonging to each of the homologous series in (a)(ii).
(iv) Name two fractions obtained from the fractional distillation of petroleum
(v) Why is there a gradual change in the physical properties of petroleum fractions?
b) Write a two-step balanced chemical equation for the reaction of (i) ethanol with excess concentration traoxosulphate (VI) acid at high temperature
(ii) excess ethanol with concentrated tetraoxosulphate (VI) acid at lower temperature.
(c) An organic compound of relative molecular mass 46, on analysis vas ound to contain 52.0% carbon, 13.3% hydrogen and 34.7% oxygen.
(i) Determine its I. empirical formula, II. molecular formula.
(ii) Draw two possible structures of the compound and name one of them [O = 16; C = 12; H = 1 ]
(a) Write an equation in each case to represent the (i) \(\beta\)-decay of \(^{24}_{11}Na\) to give Mg.
ii) reaction of sodium with cold water.
b)(i) State two differences between reaction (a)(i) and (ii)
ii) State two applications of the type of reaction represented in(a)(i)
(c) Consider the reaction represented by the equation:
Mg\(_{(s)}\) + 2HCI\(_{(aq)}\) \(\to\) MgCl\(_{(aq)}\) + H\(_{(q)}\)
(i) Name the type of reaction involved
(ii) Give two ways by which the reaction could be made faster.
(iii) What volume of hydrogen gas would be produced from 6.0 g of the magnesium? [ H = 1; 1 mole of gas occupy 22.4 dm\(^3\) at s.t.p. ]
(d) What is (i) an electrolyte?; (ii) electrolysis?
(e)(i) Give one metal that is extracted using electrolytic process.
(ii) Name the ore of the metal.
(iii) What is the substance discharged at each electrode when dilute NaCI is electrolysed using graphite electrodes?
(iv) Why would aqueous NaCI conduct electricity but solid NaCI would not?
(v) Give one industrial use of NaCI.
i) What is the name of the process used for the industrial preparation of tetraoxosulphate (VI) acid?
(ii) State the catalyst used in (a)(i)
(iii) Show by means of bdanced chemical equations only, the industrial preparation of tetraoxosulphate (VI) acid from sulphur (IV) oxide.
(b)(i) Distinguish between dehydration and drying
(ii) Explain why concentrated tetraoxosulphate (VI) acid cannot be used to dry ammonia
(iii) What is the drying agent for ammonia?
(c)(i) Give one example of I. a chloride which is soluble in hot water, II. a trioxocarbonate (IV) which does not decompose on heating, Ill. an amphoteric oxide
(ii) List three methods for the preparation of salts
(iii) State one method for the recovery of salt from its solution.
(d)(i) State Gay Lussac's law of combining volumes
(ii) Consider the reaction represented by the following equation: C\(_2\)H\(_{4(g)}\) + 3O\(_{2(g)}\) \(\to\) 2H\(_2\)O\(_{(g)}\) + 2CO\(_{2(g)}\). What is the volume of oxygen required for the complete combustion of 12.5 cm\(^{3}\) of ethene?
(i) Draw and label an energy profile diagram of an endothermic reaction. Indicate on your diagram the I. activation energy II. heat change, \(\Delta\)H.
(ii) Explain how the rate of reaction is affected by I. addition of a catalyst, II. increase in temperature.
(b)(i) Write an equation for the thermal decomposition of calcium trioxocarbonate (IV)
(ii) Determine the volume of carbon (IV) oxide measured at s.t.p. that would he produced by the thermal decomposition of 10g calcium trioxocarbonate (IV). [ Ca = 40; O = 16; C = 12 ]
(c)(i) Give one use cf each of the following forms of carbon: I. coal; II. wood charcoal; Ill. carbon (IV) oxide.
(ii) Write a balanced chemical equation to show what happens when each of the following compounds is heated strongly: I. NaNO\(_{3(s)}\) II. MgCO\(_{3(s)}\).
(d) Consider the following compounds: CaO, CaCO\(_3\), Ca(OH)\(_2\), NaOH Which of them is
(i) used in the manufacture of cement;
(ii) used to detect the presence of carbon (IV) oxide;
(iii) used to liberate carbon (IV) oxide when dilute acid is added;
(iv) hygroscopic;
(v) deliquescent?