The real harmful effect of the release of chlorofluorocarbons into the atmosphere is that it eventually causes
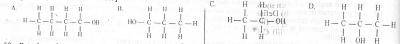
A primary alkanol has a molecular mass of 60. What is the structural formula of the compound? [C= 12.0, H= 1.0, O= 16.0)
Petroleum is a non-renewable source of energy because it
(a)(i) Sketch a graphical representation of Charles' law.
(ii) Calculate the volume of oxygen that would be required for the complete combustion of 2.5 moles of ethanol at s.t.p. [molar volume at s.t.p. = 22.4 dm\(^3\)]
(b)(i) State the collision theory of reaction rates.
(ii) Using the collision theory, explain briefly how temperature can affect the rate of a chemical reaction.
(c)(i) Define esterification.
(ii) Give two uses of alkanoates.
(iii) Give the products of the alkaline hydrolysis of ethyl ethanoate.
(d) A tin coated plate and a galvanized plate were exposed for the same length of time.
(i) Which of the two plates corrodes faster
(ii) Explain briefly your answer in 2(d)(i)
(a)(i) Draw the structure of the sixth member of the alkenes.
(ii) Calculate the relative molecular mass of the sixth member of the alkene.
(iii) State one difference between cracking and reforming in the petroleum industry. [H = 1, C = 12]
(b)(i) Define the term enthalpy of neutralization.
(ii) Describe briefly how the enthalpy of neutralization of the reaction of dilute hydrochloric acid and aqueous potassium hydroxide could be determined.
(c) An electrochemical cell is constructed with copper and silver electrodes.
(i) State which of the electrodes will be the: 1. anode; II. cathode.
(ii) Give the reason for your answer in 3(c)(i).
(iii) State the type of reaction occurring at each electrode.
(iv) Write a balanced equation for the overall cell reaction.
(d)(i) Name the compound formed when iron is exposed to moist air for a long time.
(ii) Write a balanced chemical equation for the reaction in 3(d)(i).
(iii) Name one ore of iron.