(a) State the conditions necessary for the cracking of long-chain hydrocarbons to produce more gasoline.
(b) State two reasons why metallic objects are electroplated
(c) (i) Explain briefly why calcium oxide cannot be used to dry hydrogen chloride gas.
(ii) State one drying agent for hydrogen chloride gas.
(d) Concentrated trioxonitrate (V) acid was added to a solution of iron (II) tetraoxosulphate (VI) and the mixture heated. The mixture turned from pale green to yellow with the evolution of a brown gas. Explain briefly these observations.
(e) (i) Write the equation for the reaction between zinc oxide and
(ii) State which property of zinc oxide is shown by the reaction in (e)(i).
(f) Two isotopes of chlorine are \(^{35}_{17}\)Cl and \(^{37}_{17}\)Cl State one:
(g) State the two products formed when chlorine water is exposed to sunlight.
(h) Consider the reaction represented by the following equation:
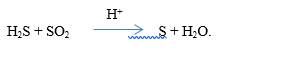
State the:
(i) What is meant by carbon-12 scale?
(j) State two properties of a chemical system in equilibrium.
(a) A hydrocarbon having the formula C\(_{10}\)H\(_{22}\) was cracked to produce C\(_6\)H\(_{14}\) and another hydrocarbon P.
(i) Give the molecular formula of P.
(ii) Draw the structures of two isomers of P.
(ii) Give a reason why P could be polymerized.
(b) State the guiding principles which are used to explain the way electrons of the atoms of the elements are arranged in atomic orbitals.
(c)Consider each of the following substances: NaH, H\(_2\), H\(_2\)S, NH\(_4\)Cl.
(i) Describe the nature of the intermolecular forces holding the units or molecules together in the condensed (liquid or solid) state.
(ii) Explain briefly what happens when a sample of each of the substances is added to water.
(iii) Write the chemical equations of any reactions occurring or of any equilibria established.
(d) Element J has the following electron configuration: Is\(^2\)2s\(^2\)2p\(^6\)3s\(^2\).
(i) How many unpaired electrons can be found in J?
(ii) State whether J would be a good oxidizing or reducing agent.
(iii) Give a reason for the answer in (d)(ii).
(a) In the Solvay process, explain briefly with equations the functions of the following substances:
(b) (i) Write a chemical equation for the fermentation of glucose.
(ii) Explain briefly why a tightly-corked glass bottle filled to the brim with fresh palm-wine shatters on standing for some time.
(c) Consider the following metals: Na, Fe, K and Cu.
(i) Arrange the metals in order of increasing reactivity.
(ii) Which of the metals will react with cold water?
(ii) Which of the metals could form coloured salts?
(d)(i) What is a redox reaction?
(ii) Identify which of the following reaction equations are redox.
(I) 2Na + Cl\(_2\) → 2NaCl
(II) AgCl + 2NH\(_3\) → [Ag(NH\(_3\))\(_2\)]Cl
(III) C\(_2\)H\(_2\) + H\(_2\) → C\(_2\)H\(_4\)
(IV) HCl + KOH → KCl + H\(_2\)O
(V) 2FeCl\(_3\) + 2KI → 2FeCl\(_2\) + 2KCl + I\(_2\)
(iii) Give a reason for each of the answers in (d)(ii).
(iv) Write balanced equations of the half reactions for any two of the redox reactions in (d)(ii).
(a) The following reaction scheme is an illustration of the contact process. Study the scheme and answer the questions that follow.
(i) Name X and Y
(ii) Write a balanced chemical equation for each of the processes I, II, III and IV
(iii) Name the catalyst used in process II
(iv) Using Le Chatelier's principle, explain briefly why increasing the temperature would not favour the reaction in II
(v) State two uses of SO\(_2\)
(b) Consider the following equation: 2H\(_{2(g)}\) + O\(_{2(g)}\) \(\to\) 2H\(_2\)O\(_{(g)}\)
Calculate the volume of unused oxygen gas when 40 cm\(^3\) of hydrogen gas is sparked with 30cm\(^3\) of oxygen gas
(c) Calcium carbonate of mass 1.0 g was heated until there was no further change.
(a)(i) Draw and label a diagram to illustrate the preparation and collection of dry chlorine gas in the laboratory.
(ii) State two uses of chlorine.
(b) Describe the preparation of hydrogen from water gas.
(i) Name the chief ore of aluminium.
(ii) Why is the ore purified?
(iii) Name the electrode used in the electrolysis.
(iv) Give one reason why cryolite, NaAlF\(_6\), is added to the electrolyte.
(c) Name three products obtained directly from the destructive distillation of coal.