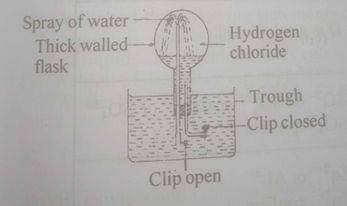
(a) Consider the following diagram ABOVE.
(i) Give the name of the experiment.
(ii) What does the experiment demonstrate?
(iii) Name one gas that could be used in place of HCl gas.
(iv) What colour could be observed in the flask during the spray of water?
(v) Could the gases used in the experiment be collected over water?
(vi) Explain briefly your answer in (a)(v).
(b) A solid substance U when strongly heated decomposes to give a white solid V and carbon (IV) oxide. When water is added to V, W is produced W can be used to test for carbon (IV) Oxide. Identify U, V and W.
Credit will be given for strict adherence to the instructions, for observations precisely recorded and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C and D are inorganic salts. E is a solution of an organic compound. Carry out the following exercises on C, D and E. Record your observations and identify any gas(es) evolved. State the conclusion you draw from the result of each test.
(a) To all of C, add about 2 cm\(^3\) of distilled water in a boiling tube. Shake to dissolve and test the solution with litmus paper.
(b)i) Test E with litmus paper.
(ii) Add all of E to the solution obtained in (a).
(c)(i) Add about 5 cm\(^3\) of distilled water to D in a test tube and shake thoroughly. Divide the resulting solution into two portions.
(ii) To the first portion, add few drops of BaCl\(_{2(aq)}\) followed by excess dil. HCl.
(iii) To the second portion, add HNO\(_{3(aq)}\) in drops and then in excess.
All your burette readings (initials and final) as well as the size of your pipette must be recorded but no account experimental procedure is required. All calculations must be done in your answer booklet.
A is 0.100 mol dm\(^{-3}\) HNO\(_3\). B is a solution containing 2.50 g of a mixture of Na\(_2\)CO\(_3\) and Na\(_2\)SO\(_4\) in 250 cm\(^3\) of solution.
(a) Put into the burette and titrate it against 20.0 cm\(^3\) or 25.0cm\(^3\) portions of B using methyl orange as indicator. Repeat the exercise to obtain consistent titre values. Tabulate your readings and calculate the average volume of acid A used. The equation of reaction is Na\(_2\)CO\(_{3(aq)}\) + 2HNO\(_{3(aq)}\) \(\to\) NaNO\(_{3(aq)}\) + H\(_2\)O\(_{(l)}\) + CO\(_{2(g)}\)
(b) From your results and the information provided, calculate the:
(i) concentration of B in moldm\(^{-3}\);
(ii) concentration of Na\(_2\)CO\(_3\) in B in gdm\(^{-3}\);
(iii) percentage of Na\(_2\)CO\(_3\), in the mixture. [Na\(_2\)CO\(_3\) = 106]