(a)(i) Define the term hygroscopic.
(ii) Give two difference: between a physical change and a chemical change.
(iii) Using the kinetic theory of gases, explain briefly the Charles' law.
(b)(i) Arrange the following compounds in order of increasing boiling points: CS\(_2\); CO\(_2\); NaH. Give reasons for your answer.
(ii) Write a balanced chemical equation to illustrate the reaction of chlorine gas with cold dilute sodium hydroxide.
(c) In a certain reaction, 15.0 g of impure magnesium sample reacted with excess hydrochloric acid liberating 8.6 dm\(^2\) of hydrogen gas at s.t.p.
(i) Write a balanced equation for the reaction.
(ii) Calculate the: I. mass of pure magnesium in the sample; I. percentage purity of the magnesium sample; III. number of ions produced in the reaction. [Mg = 24.0; volume at s.t.p. 22.4 dm\(^{-3}\), Avagadro's constant = 6.02 x 10\(^{23}\)mol\(^{-1}\)]
(a)(i) Explain briefly the term chemical industry.
(ii) State three factors that should be considered in siting a chemical industry.
(b)(i) Describe briefly twin is extracted from its ore.
(ii) Give two uses of tin.
(c)(i) Name the constituents of cement.
(ii) How does mortar set?
(d)(i) Explain briefly the term pollution.
(ii) Give two examples of air pollutants.
(e) Consider the following reversible reaction which occurred at the temperature of 298K:
N\(_{2(g)}\) + 3H\(_{2(g)}\) \(\rightleftharpoons\) 2NH\(_{3(g)}\); \(\bigtriangleup\)H = —92.37kJ
(a)(i) List two characteristics of homologous series.
(ii) Consider the compound represented by the following formula: CH\(_3\)(CH\(_2\))\(_2\)CH\(_3\). I. Which homologous series does the compound belong? II. Write the structures of three possible isomers of the coumpound. III Name the three possible isomers in (a)(ii)II.
(b) Write the structure of the major product formed in each of the following reactions: (i) ethanol with excess acidified potassium tetraoxomanganate (VII);
(ii) excess ethane with chlorine in the presence of sunlight;
(iii) ethanol with propanoic acid in the presence of few drops of concentrated tetraoxosulphate(VI) acid.
(c) Name the major product in each reaction in (b).
(d) Consider the following organic compounds:
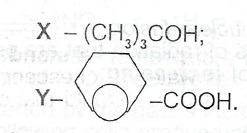
(i) Give the IUPAC name of each compound.
(ii) State a chemical test for the functional group in each compound.
(e) An organic compound with relative molecular mass 136 contains 70.57% carbon, 5.90% hydrogen and 23.53% oxygen. Determine its: (i) empopirical formula; (ii) molecular formula. [H= 1.00, C= 12.0, H = 16.0]
(a)i) How would an aqueous solution of iron (II) tetraoxosulphate (VI) be converted into an aqueous solution of magnesium tetraoxosulphate (VI)?
(ii) Write a balanced equation for the reaction in (a)(i) above
(b)(i) Why are some compounds recrystallized after preparation?
(ii) Outline the steps in recrystallization
(C)(i) Name two gases that can cause color changes in an acidified solution of potassium heptaoxodichromate (VI)
(ii) State the color change expected in (c)i) above
Credit will be given for strict adherence to the instructions, for observations precisely recorded, and for accurate inferences. AIl tests. observations and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C is a double salt. Carry out the following exercises on C. Record your observations and identify any gas(es) evolved. State the conclusion drawn from the result of each test.
a) Put all of C into a test tube. Add about 5cm\(^3\) of distilled water, stir and test with litmus paper. Divide the Solution into two portions
(b) To the first portion, add sodium hydroxide solution in drops and then in excess. Heat the resulting mixture and keep it for minutes.
(c) To the second portion, add few drops of Bacl\(_{2(aq)}\) followed by excess dilute hydrochloric acid