(a) What is the shape of (i) p - orbital; (ii) a molecule of methane; (iii) a molecule of carbon (IV) oxide?
(b) Consider the following elements: Ne, S, CI, 0, Fe, Mg. State which of them
(i) exhibit(s) allotropy;
(ii) form(s) coloured ions;
(iii) is/are malleable;
(iv) consist(s) of molecules that are far apart at room temperature;
(v) form(s) hydrides by sharing electrons with hydrogen;
(vi) has/have complete outermost shell.
(c)(i) List three applications of radioactivity in different fields.
(ii) Explain clearly the difference between the following reactions involving electron loss from lead.
\(^{211} pb\) \(\to\) \(^{ 211}Bi\) + \(^0_{-1}\); Pb \(\to\) pb\(^{3+}\) _ 2e\(^-\)
(iii) Give one advantage and one disadvantage of nuclear power generation over the use of fossil fuels.
(a) Giving different examples, mention one metal in each case which produces hydrogen on reacting with
(i) dilute mineral acid
(ii) cold water;
(iii) steam;
(iv) hot, concentrated alkali.
(b) In an experiment, excess 0.50 mol dm\(^{-3}\) HCI was added to 1Og of granulated zinc in a beaker. Other conditions remaining constant, state how the reaction rate would be affected in each case, if the experiment was repeated using:
(i) 1.0 mol dm\(^{-3}\) HCI;
(ii) 8.0g of granulated zinc;
(iii) 10g of zinc dust;
(iv) a higher volume of 0.50 mol dm HCI;
(v) a reaction vessel dipped in crushed ice;
(vi) equal volumes of water and 0.50 mol dm\(^3\) HCI.
(c) Aluminium is extracted from its ore by electrolysis.
(i) Name the ore from which the metal is extracted.
(ii) State the role of molten cryolite in the extraction.
(iii) Describe in outline how the ore is purified before electrolysis
(iv) Calculate the current in amperes required to produce 18.0g of aluminium in 1.50 hours. [Al = 27.0; F = 96500C]
(d) Give the reason why
(i) aluminium, which is a reactive metal, is resistant to corrosion.
(ii) metals are generally good reducing agents.
(a) What term is used to describe each of the following processes?
(i) Alkaline hydrolysis of fats and oils;
(ii) The conversion of glucose into ethanol by enzymatic action;
(iii) Thermal decomposition of higher petroleum fractions into lower molecular mass hydrocarbons in the presence of catalyst.
(b)(i) Write the structure and IUPAC name for one alkanoic acid with the molecular formula C\(_4\)H\(_8\)0\(_2\).
(ii) Arrange the following compounds in order of increasing boiling point: Butane; Butanoic acid; Methylpropane.
(iii) Give an explanation for your answer in (b)(ii).
(c)(i) Ethanol was used for preparing a gas X which decolorized bromine water. Identify X and describe briefly its laboratory preparation.
(ii) Write an equation to show how ethanol reacts with sodium
(iii) Give the reagent and reaction conditions for the conversion of ethanol into C\(_2\)H\(_5\)COOC\(_2\)H\(_5\).
(d) State the type of recction involved in each of the conversions indicated below:
(i)C\(_6\)H\(_6\)C\(_6\)H\(_5\)CH\(_3\)
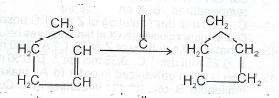
(ii) nC\(_2\)H\(_4\) \(\to\) (CH\(_2\) - CH\(_2\)),
(iii) CH\(_3\)CH\(_2\)CH(OH)CH\(_3\) -> CH\(_3\)CH\(_2\)CCH\(_3\)
(iv) (C\(_6\)H\(_{10}\)O\(_5\)) -> C\(_6\)H\(_{12}\)O\(_6\).
(iv)