(a) If a steel spoon were to be plated with silver, state what would be suitable for use as the;
(i) anode. (ii) cathode; (iii) electrolyte
(b)(i) Write an equation for one of the reactions involved in the purification of bauxite.
(ii) Give the reason why the carbon anodes are changed at intervals during the electrolysis of pure alumina solution in molten cryolite.
(a)(i) List the quantum number that are assigned to an electron in an atom.
(ii) What is the maximum number of electrons that can occupy the 3d orbital?
(b) An element represented as P has the following electronic configuration: 1s\(^2\)2s\(^2\)2p\(^6\)2s\(^2\)
(i) Write the electronic configuration of the ion of P.
(ii) Without identifying P, write the likely formula of its chloride.
(iii) State with reason, whether P will be a good oxidizing or reducing agent.
(c)(i) What is electron affinity?
(ii) Explain briefly why ammonia can precipitate in dative bonding.
(d)(i) If an element in Group IV loses an alpha particle, to which group would the product belong?
(ii) Two equally toxic substances X and Y which decay to non-toxic products, were absorbed through the skin. If their half-lives are 8 minutes and 2 months respectively, which of them constitutes the greater health hazard? Explain your answer
(a)(i) What type of reaction is involved in each of the conversion processes indicated as I to V below?
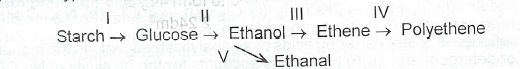
(ii) Name one isomer of glucose
(iii) Explain why palmwine becomes sour on prolonged exposure to air.
(b)(i) List the reagents and the reaction condition necessary for ethanoic acid to form an alkanoate.
(ii) Give two uses of alkanoates
(c)(i) What is the lUPAC name of the following compound?
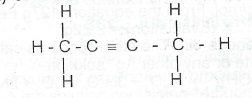
(ii) Outline one chemical test to distinguish between methane and the compound in (c)(i) above.
(iii) Write an equation for the combustion of ethene in excess oxygen.
(a)(i) Define heat of neutralization
(ii) Give the reason why copper (II) chloride can be prepared by neutralization, unlike lead (II) chloride.
(b)(i) Describe in outline, the manufacture of trioxonitrate (V) acid by the catalytic oxidation of ammonia, giving equations where appropriate.
(ii) What are the products obtained when sodium tioxonitrate (V) is heated strongly?
(c) When powdered magnesium is heated to redness in a stream of nitrogen, magnesium nitride (Mg\(_3\)N\(_2\)) is formed.
(i) Write an equation for the reaction
(ii) Hence, calculate the amount (in mole) of magnesium nitride that can be obtained from 3.0g of magnesium [Mg = 24].
(a)(i) What is an electrolyte?
(ii) Classify each of the following as strong electrolyte/weak, electrolyte/non-electrolyte. Potassium chloride; sodium ethanoate, aqueous ammonia; cane sugar
(b)(i) Write half-cell equations for the reactions in the Daniel cell .
(ii) Why is the Daniel cell classified as an electrochemical cell?
(iii) Give two other examples of electrochemical cell.
(c) Explain the following observations.
(i) Graphite conducts electricity, unlike most non-metals
(ii) In the electrolysis of copper (II) tetraoxosulphate (VI) solution, the blue colour fades with platinum electrodes while the colour intensity is unaffected with copper electrodes (equations required)
(iii) A solution of dry hydrogen chloride in methylbenzene (toluene) does not conduct electricity whereas hydrochloric acid does.