(a) Mention the chemical substance manufactured starting from each of the foirownc sets of materials:
(i) sugar and yeast;
(ii) ammonia, air and water;
(iii) vegetable oil and caustic alkali.
(b) State one air pollutant generated during the manufacture of fertilizers.
(a) What is the change in oxidation state of chromium in the reaction represented by the following equation?
3SO\(_2\) + Cr\(_2\)O\(^2_{-7}\) + 2H\(^+\) -> 3SO\(_4^{2-}\) + 2Cr\(^{3+}\) + H\(_2\)O
(b) Use the half equations given below to deduce the equation for the reaction between iron(II) ions and heptaoxodichromate (VI) ions in acidic solution.
Fe\(^{2+}\) --> Fe\(^{3+}\) + e\(^-\)
Cr\(_2\)O\(^{2-}_7\) + 14H\(^+\) + 6e\(^-\) ----> 2Cr\(^{3+}\) + 7H\(_2\)O.
(a) Mention one process apart from respiration, which increases the amount of carbon (IV) oxide in the atmosphere.
(b)(i) State one use of sodium hydrogentrioxocarbonate (IV).
(ii) Write an equation to show the action of heat on sodium hydrogentrioxocarbonate(IV).
(a) List two differences between solids and liquids.
(b) The graph below is the heating curve for a solid X. Use the graph to answer Questions (i) — (iii) below.
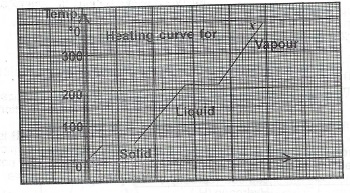
(i) What is the melting point of X?
(ii) If the vapour of X is cooled, at what temperature will it start to condense?
(iii) (I) As X is heated, state what happens to the: I. frequency of collision of molecules of X;
(II) value of the entropy of the system.
(a)(i) List the three types of particles present in atoms.
(ii) name the element which does not contain all the three particles in its atom. Mention the particle that is not present.
(b) Give the reason why:
(i) the relative atomic masses of some elements are not whole number;
(ii) relative atomic masses are used instead of the actual masses of atoms in grams;
(iii) metals are good conductors of electricity.
(c)(i) Name the type of bond present in the oxonium ion,
(ii) State one effect of the existence of intermolecular hydrogen bonding on the physical properties of ethanol.
(d)(i) Explain what is meant by water of crystallization.
(ii) When 5.0g of a compound Y was heated to constant mass, 1.8g of water vapour was given off. Determine the number of molecules of water of crystallization in one molecule of Y, given that the molar mass of its anhydrous form is 160g. [H = 1, 0 = 16]