(a) What is meant by the term acid salt? Give one example.
(b) State the reason why an all-glass apparatus must be used for the laboratory preparation of concentrated trioxonitrate (V) acid.
(a) Name: (i) one structural isomer of glucose.
(ii) the process by which starch is converted to glucose.
(b) The open-chain structure of glucose is shown below.
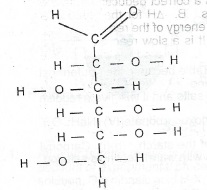
(a) State the functional groups present in the structure.
(ii) Which of the functional groups would react with warm Fehling's solution?
(a)(i) State three differences between electrovalent compound and covalent compound.
(ii) Name the type of chemical bonding involved in the formation of ammonium ion from ammonia.
(b)(i) Name the quantum numbers which define an electron within an atom.
(ii) State the orbital in which the fifth electron of an atom is most likely to be found. Sketch the shape of the orbital.
(iii) State the period and the group to which the element boron belongs in the Periodic Table.
(c)(i) What is meant by the entropy of a chemical system?
(ii) Calculate the free energy change for a given reaction at 300 K using the following data obtained for the reaction: \(\Delta\) = -710KJ mol\(^{-1}\); \(\Delta\)S = 0.15 KJ mol\(^{-1}\)K\(^{-1}\)
(iii) From your evaluation in (c)(i) above, state whether the reaction is spontaneous or not at the given temperature. Give reason for your answer.
(a) Give the products of the following reactions:
(i) hydrolysis of simple proteins.
(ii) alkaline hydrolysis of fats and oils.
(b) A combustion tube was packed with small pieces of broken clay pot and the tube maintained at a temperature of 750K. When the vapour of decane was passed into the tube, the main products included a gaseous hydrocarbon X.
(i) Name the process involved in the reaction. Give its industrial application.
(ii) State the function of the pieces of broken pot in the experiment.
(iii) Give one chemical test to distinguish between X and methane.
(iv) Draw a labelled diagram for the laboratory preparation of X.
(c)(i) State what would be observed if a piece of sodium was added to 10cm\(^3\) of propanol in a beaker. Write an equation for the reaction.
(ii) Give the main product formed when excess acidified potassium heptaoxodichromate (VI) reacts with each of the following: propan-1- ol: propan - 2 -of; State the type of process involved in the reactions.
(a)(i) State Graham's law of diffusion.
(ii) Consider the reaction represented by the following equation:
N\(_2\)O\(_{4(g)}\) 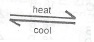 2NO\(_{2(g)}\)
2NO\(_{2(g)}\)
Night yellow dark brown
State what would happen to the vapour density of N\(_2\)O\(_4\) as the temperature of the system is increased. If the system is cooled, would the gases become lighter or darker in colour? Explain your answer in each case.
(b) Explain the following observations:
(i) an inflated balloon that was left in the sun. burst after some time;
(ii) a pure sample of a liquid did not have a constant boiling point at the top and at the base of a high mountain
(c)(i) List two gaseous reducing agents
(ii) Write one equation each to illustrate the reducing property of the gases you listed in (c)(i) above.