(a) Name the type of solid structure possessed by:
(i) diamond;
(ii) iodine;
(iii) sodium chloride.
(b) Give:
(i) one alloy of tin:
(ii) a common reducing agent which is a compound of tin.
Liquefied air is mainly a mixture of nitrogen and oxygen which can be separated into its components by fractional distillation (Boiling point of nitrogen is 196°C, Boiling point of oxygen is 174°C)
(a) Name the fraction which distills over first. Give the reason for your answer.
(b) Give another industrial application of fractional distillation as a separation technique.
(a)(i) List two physical properties used as criteria for purity of substances
(ii) describe how you would prepare a pure, dry sample of sodium chloride crystals by a neutralization reaction, using bench reagents.
(iii) Give two other general methods for preparing soluble salts.
(b) Explain the following observations:
(i) a sheet of iron placed in dilute copper (II) tetraoxosulphate (VI) solution reddish brown;
(ii) the white gelatinous precipitate formed when a few drops of sodium hydroxide solution are added to a solution of aluminium salt dissolves in excess alkali;
(iii) the pale green prepared iron(II) chloride solution changes to brown on bubbling chlorine gas through it.
(iv) Write a balanced equation for the reaction of dilute hydrochloric acid with marble. List two industrial process in which limestone is used as a raw material.
Consider the reaction represented by the equation: 2SO\(_{2(g)}\) + O\(_{2(g)}\) 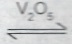 2SO\(_{3(g)}\). \(\Delta\)H = 188KJ.
2SO\(_{3(g)}\). \(\Delta\)H = 188KJ.
(a) Write an expression for the equilibrium constant
(b) Sketch an energy diagram for the forward reaction, showing the profile for the catalyzed and non-catalyzed systems.
(c) state the reason, the effect of the following on the position of equilibrium of the system:
(i) increase in temperature
(ii) increase in pressure;
(iii) removal of some of the SO\(_3\) produced;
(iv) presence of V\(_2\)O\(_5\)
(d)(i) Write equations to show how the sulphur(VI) oxide is converted to tetraoxosulphate(VI) acid in the contact process
(ii) Give two uses of tetraoxosulphate(VI) acid.
(a)(i) Define the term addition polymerization
(ii) What type of organic compounds undergo addition polymerization
(iii) List two factors which affect the strength of polymers
(b) The diagram below shows some reaction pathways involving ethanol
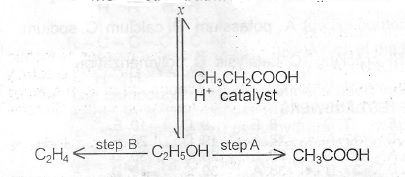
(i) Write the name and structural of the organic product X
(ii) State the reagent for the conversation indicated as step A.
(iii) What type of reaction will ethanol undergo CH\(_3\)CH\(_2\)COOH during the process of conversation indicated as step B?
(c)(i) Write three balanced equation for the complete combustion of ethanol in :date the volume of oxygen required at s.t.p for the complete combustion of ethanol. (H = 1, C = 12, O = 16, molar volume of gases at s.t.p. = 22.4 dm\(^3\))
(d)( i} State two substances produced when coal is heated in the absence of air
(ii) What name is given to the process in (d)(i) above?
(iii) State the importance of the non-volatile residue of the process named in (d)(iii) to the iron and steel industry.