If a reaction is exothermic and there is a great disorder, it means that?
In the preparation of oxygen by heating KClO3 in the presence of MnO2, only moderate heat is needed because the catalyst acts by?
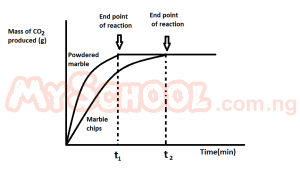
The graph above demonstrate the effect of?
2H2(g) + O2(g) ⇌ 2H2O(g) ΔH = -ve
what happens to the equilibrium constant of the reaction above if the temperature is increased?
To a solution of an unknown compound, a little dilute tetraoxosulphate (VI) acid was added with some freshly prepared iron (II) tetraoxosulphate (VI) solution. The brown ring observed after the addition of a stream of concentrated tetraoxosulphate (VI) acid confirmed the presence of?