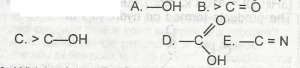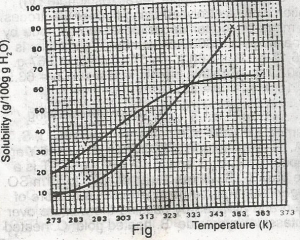
The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If the molar mass of X is 36 g, the number of moles of X dissolved at 343 K is
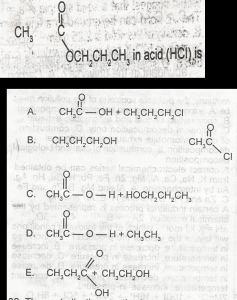
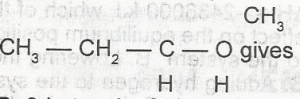
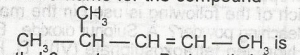
The I.U.P.A.C name for the compound