(a) Explain with equation where appropriate, the functions of the following substances in the Solvay Process:
(i) limestone,
(ii) ammonia,
(iii) brine.
(b) Explain why the reaction between aqueous sodium trioxocarbonate (IV) solution and dilute hydrochloric acid is a neutralization reaction.
(c) Calculate the mass of sodium trioxocarbonate (IV) produced by the complete decomposition of 16.8g of sodium hydrogen trioxocarbonate (IV) (H = 1, O = 16, Na = 23, S = 33)
(a)(i) Limestone is a cheap and available raw material for the solvay process. It is heated to decompose to produce carbon(IV) oxide, an important ingredient in the solvay reaction i.e CaCO\(_{3(S)}\) \(\to\) CaO\(_{(S)}\) + CO\(_{2(g)}\)
(ii) Ammoninia dissolved in water will give aqueous ammonia which constitute an important reactant in the solvay process.
(iii) Sodium chloride is the rich source of sodium which is one of the composing elements of NaHCO\(_3\), a main product of the solvay.process
i.e
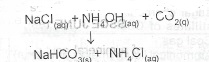
(b) The hydrolysis reaction of sodium trioxocarbonate (IV) produces a basic medium
i.e 2Na\(^+\).CO\(^{2-}_3\) + 2H\(^+\)2OH\(^-\) -> 2NaOH + H\(_2\)CO\(^3\). Hence the reaction between dilute hydrochloric acid and sodium trioxocarbonate (IV) solution can be said to be acid-base reaction which is also neutralisation reaction.
(c) 2NaHCO\(_{3(S)}\) ---> Na\(_2\)CO\(_{3(s)}\) + H\(_{2}\)O\(_{(g)}\) + CO\(_{2(g)}\)
168g produced 106g Na\(_2\)CO\(_3\)
i.e lg will produce \(\frac{106}{168}\)
16.8g will produce \(\frac{106}{168} \times \frac{16.8}{1}\)
= 10.6g Na\(_2\)CO\(_3\)
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}