(a)(i) Define the term addition polymerization
(ii) What type of organic compounds undergo addition polymerization
(iii) List two factors which affect the strength of polymers
(b) The diagram below shows some reaction pathways involving ethanol
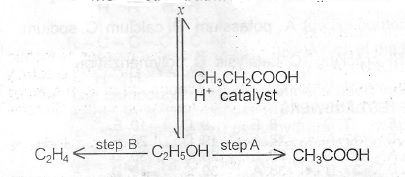
(i) Write the name and structural of the organic product X
(ii) State the reagent for the conversation indicated as step A.
(iii) What type of reaction will ethanol undergo CH\(_3\)CH\(_2\)COOH during the process of conversation indicated as step B?
(c)(i) Write three balanced equation for the complete combustion of ethanol in :date the volume of oxygen required at s.t.p for the complete combustion of ethanol. (H = 1, C = 12, O = 16, molar volume of gases at s.t.p. = 22.4 dm\(^3\))
(d)( i} State two substances produced when coal is heated in the absence of air
(ii) What name is given to the process in (d)(i) above?
(iii) State the importance of the non-volatile residue of the process named in (d)(iii) to the iron and steel industry.
(a)(i) Addition polymerization is the combination of many molecules of the same kind to form a complete molecule without gain or loss of material.
(ii) Manomers which contain one or more double bonds usually undergo addition polymerization.
(iii) Must be of high molecular weight and must be softened at high possible temperature.
(b)(i) Propylethanoate (CH\(_3\)CH\(_2\)COOCH\(_2\)CH\(_3\))
(ii) Reagent indicated at Step A potassium heptaoxodichromate(VI) in dilute tetraoxosulphate(VI) acid.
(iii) Dehydration reaction.
(c)(i) CH\(_3\)CH\(_2\)OH\(_{(aq)}\) + O\(_{2(g)}\) \(\to\) CH\(_3\)COOH\(_{(l)}\) + H\(_2\)O\(_{(l)}\)
(ii) At s.t.p 46g CH\(_3\)CH\(_2\)CH\(_2\)OH was completely burnt by 22.5dm\(^3\)
i.e. 1g CH\(_3\)CH\(_2\)OH was completely burnt by \(\frac{32.4dm^3}{46g}\)
2.3g CH\(_3\)CH\(_2\)OH was completely burnt by \(\frac{22.4dm^3}{46}\) x 2.3g = 1.12dm\(^3\)O\(_2\)
distillation of coal and it is important in the reduction of iron to the element iron.
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}