Consider the reaction represented by the equation: 2SO\(_{2(g)}\) + O\(_{2(g)}\) 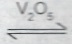 2SO\(_{3(g)}\). \(\Delta\)H = 188KJ.
2SO\(_{3(g)}\). \(\Delta\)H = 188KJ.
(a) Write an expression for the equilibrium constant
(b) Sketch an energy diagram for the forward reaction, showing the profile for the catalyzed and non-catalyzed systems.
(c) state the reason, the effect of the following on the position of equilibrium of the system:
(i) increase in temperature
(ii) increase in pressure;
(iii) removal of some of the SO\(_3\) produced;
(iv) presence of V\(_2\)O\(_5\)
(d)(i) Write equations to show how the sulphur(VI) oxide is converted to tetraoxosulphate(VI) acid in the contact process
(ii) Give two uses of tetraoxosulphate(VI) acid.
(a) Equilibrium constant (Kc) = \(\frac{[SO_3]}{[SO]^2 \times [O_2]}\)
(b) The reaction profile; 2SO\(_{2(g)}\) + O\(_{2(g)}\) \(\to\) 2SO\(_{3(g)}\)

(c)(i) (i) Increase in temperature low of SO\(_{3(g)}\) because the contact proc Decrease in temperature favour E more SO\(_{3(g)}\).
(ii) Increase in pressure favour the more production of SO\(_{3(g)}\) because of moles is present in the prod reactant side of the overall read process.
(iii) Removal of some of the SO\(_{3(g)}\) produced will favour the forward flow of the Chatelier's principle about Equilibrium
(iv) Presence of V\(_2\)O\(_5\), it acts as a fasten the more production SO\(_{3(g)}\) temperature.
(d) Step I: 2SO\(_{2(g)}\) + O\(_{2(g)}\) \(\to\) 2SO\(_{3(g)}\) (Contact process)
Step II: H\(_2\)SO\(_{4(aq)}\) + SO\(_{2(g)}\) \(\to\) 2H\(_2\)S\(_2\)O\(_{7(aq)}\) Oleum fuming liquid conc
Step III: H\(_2\)S\(_2\)O\(_{7(aq)}\) + 2H\(_2\)O\(_{(l)}\) \(\to\) 2H\(_2\)SO\(_{4(aq)}\) tetraoxosulphate (VI) acid
(ii) Tetraoxosulphate (IV) acid can be used for the manufacture of paints and pigment. It can also be used for making detergents and soap.
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}