(a)(i) State two differences betwecii the properties of solids and gases
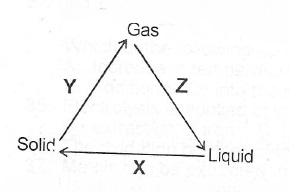
(ii) What process does each of X, Y and Z represent in the changes shown below?
(b)(i) State Charles' Law (ii) Draw a sketch to graphically illustrate Charles' Law.
(c) 60 cm of hydrogen diffused through a porous membrane in 10 minutes. The same volume of a gas G diffused through the same membrane in 37.4 minutes. Determine the relative molecular mass of G. [ H = I ]
(d)(i) State two assumptions X of the kinetic theory.
(ii) Consider the reaction represented by the Solid of Liquid following equation:
H\(_{2(g)}\) + Cl\(_{2(g)}\) \(\to\) 2HCI\(_{(g)}\)
Use the kinetic theory to explain how the rate of formation of HCI\(_{(g)}\) would be affected by I. increase in temperature; II. decrease in pressure.
(e) Given different examples, mention one metal in each case vihich produces hydrogen on reacting with (i) dilute mineral acid; (ii) cold water; (iii) steam; (iv) hot, concentrated alkali.
(a)(i)
| Solids | Gases |
|
Particles are held together rigidly by strong forces (of attraction) |
Negligible force (of attraction) between particbs |
|
Particles vibrate at fixed position. |
Particles are in constant motion. |
|
They cannot be compressed easily. |
They are highly compressible |
|
They have definite shape |
They have no definite shape. |
|
Solids do not expend appreciably on heating |
They rapidly expand on heating. |
(ii) X is freezing Y is sublimation Z is condensation.
(b)(i) Charles' law states that at a constant pressure, the volume of a given mass of gas directly proportional to its absolute temperature
(ii)

(c) \(\frac{r_1}{r_2} = \frac{d_2}{d_1} = \frac{r_H}{r_G} = \sqrt{M_G}{M_H}\)
= \(\frac{\frac{60}{10}} {\frac{60}{10}}\) = \(\frac{M_G}{2}\)
\(\frac{6}{1.6}\) = \(\frac{M_G}{2}\) = 3.74
= \(\frac{M_G}{2}\)
\(M_G\) = 13.99 x 2 = 27.97
\(M_G\) = 27.97
Relative molecular mass = 28
(d)(i) (1) Gases are composed of discrete particles called molecules, which are in rapid, random motion.
(2) Collision occurs between the molecules themselves and between the molecules and the wall of the containing vessel.
(3) The distance between the molecules is large in comparison to the size of the molecules.
(ii) H\(_{2(g)}\) + Cl\(_{2(g)}\) \(\to\) 2HCL\(_{(g)}\)
(1) Increase in temperature increases the kinetic energy of the particles, resulting in increased collision leading to increase in effective collision hence the rate of reaction increases.
(iii) Decrease in pressu-re leads to an increase in volume of the particles, which make them further apart, resulting in fewer collisions, leading to fewer effective collision and hence the rate of reaction decreases.
(e) (i) dilute mineral acid --> Mg/Fe/Sn/Zn/Pb
(ii) cold water Ca/K/Na
(iii) steam --> Fe/Zn/Mg/AL
(iv) hot conc. alkali ---> AL/Zn/Pb
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}