a) Define each of the following terms and indicate one use of each:
(i) Nuclear fission; (ii) Nuclear fusion.
(b) Alpha particle emission by \(^{293}_{25}U\) proceduces an element A. Beta particle emission by the particle A produces another element B. Element B also undergoes alpha particle emission to produce \(^{227}_{89}AC\). Write balanced equations to represent the above statement.
(c) The models below represent the filling of orbitals in an atom.
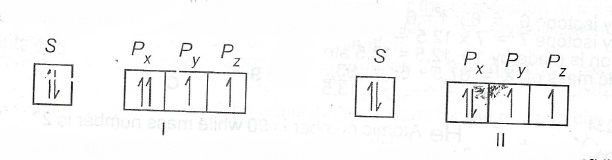
State which rule(s) is/are violated or obeyed by each model.
(d) Explain why the boiling point of H\(_2\)S with relative molecular mass of 34 is lower than that of H\(_2\)O with relative molecular mass of 18.
(e) HCI is passed into each of the following solvents:
(i) water;
(ii) methylbenzene. I. State the effect of each solution on blue litmus paper II. Compare the electrical conductivities of the two solutions.
(f) Zinc dust is added to copper (II) tetraoxosulphate (VI) solution. State;
(i) what is observed; (ii) the type of reaction that occurs.
(a) (i) Nuclear fission: is the splitting of a heavy atomic nucleus into two approximately equal fragments, with evolution of large amount of energy. Use of nuclear fission: It is used for generating large amount of energy.
(ii) Nuclear fusion: Is the process whereby two light nuclei combine to form a heavier nucleus with energy generated or released. Use of nuclear fusion: It is used for generated radioactive isotopes.
(b) \(^{235}_{92}U \to ^{231}_{90}A + ^4_2\alpha\)
\(^{231}_{90}U \to ^{231}_{91}\beta + ^0_{-1}\alpha\)
\(^{231}_{91}B \to ^{227}_{89}AC + ^4_2\alpha\)
Accept \(^{4}_{2}\)He as \(\alpha\)—particle and \(^o_{-1}e\) as \(\beta\)— particle
(c) Model I: Obeys Aufbaus and Hund's, violates Pauli's Exclusion Principle. Model II: Obeys all the three rules i.e Hund, Aufbaus and Pauli.
(d) In general, the higher the molar mass, the higher the boiling point. Oxygen is more electronegative than sulphur. H\(_{2}\)O molecules are held together by stronger hydrogen bond than in H\(_2\)S. Hence, more energy is required to break the hydrogen bond in water.
(e)(i)HCI in water turns blue litmus to red. HCI in methyl benzene has no effect on blue litmus paper.
(ii) The methyl benzene solution does not conduct electricity while HCI in water conducts electricity.
(f) (i) Drown precipitate is observed and the blue colour fades
(ii) The reaction is a redox or displacement Solids reaction. Gases
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}