(a)Define the term solubility.
(b) The table below gives the solubility of salt Z at various mperatures
|
|
0 | 10 | 20 | 30 | 40 | 50 | 60 |
|
Solubility (mol dm\(^{-3}\)) |
0.13 | 0.21 | 0.31 | 0.45 | 0.63 | 0.85 | 1.10 |
(i) Plot a graph of solubility against temperature.
(ii) From the graph determine the solubility of salt Z at 35°C.
(iii) If 100cm\(^{-3}\) of the saturated solution is cooled from 55°C to 35°C, calculate the mass of salt Z that would crystallize out. [Molar mass of salt Z = 100 g]
(c)(i) Write a balanced equation to illustrate the reaction of AI\(_2\)O\(_3\) with dilute I. HCI; II. NaOH.
(ii) What is the name given to an oxide that exhibits both acidic and basic properties?
(iii) Give one metallic oxide which exhibite these properties.
(d)(i) Determine the oxidation number of: I. Al in [Al (H\(_2\)O)\(_6\)]\(^{3+}\); II. H in NaH.
(ii) Give the IUPAC name of each of the following substances; I. CuSO\(_4\).5H\(_2\)O; II. CaCO\(_3\); Ill. KMnO\(_4\).
(a) Solubility is the maximum amount of solute that would dissolve / be contained in 1 dm\(^3\) of solution at a given temperature.
(b)(i)
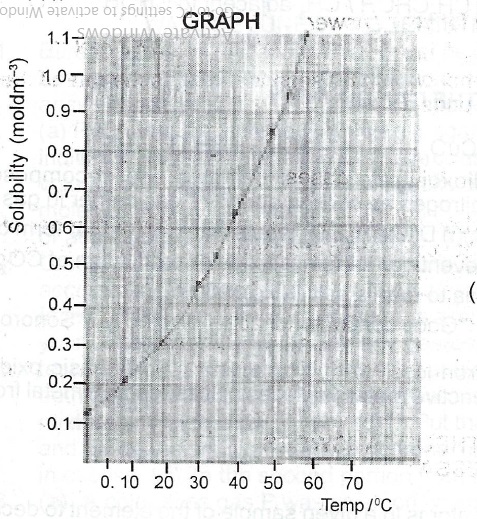
(ii) At 35° solubility = 0.50 to 0.55 moldm\(^{-3}\) range
(iii) Solubility @ 55°C = 0.95 to 0.98 moldm\(^{-3}\)
1 moldm\(^{-3}\) of salt Z = 0.97; 100 = 97g
100cm\(^{-3}\) will contain = \(\frac{97 \times 100}{100}\) = 9.7g
Solubility @ 35°C = 0.54moldm\(^{-3}\)
1 drn\(^{-3}\) contain = 0.54 x 100 = 54g
100cm\(^{-3}\) will contain 54 x100 = 5.4g
10 Mass of salt that would crystallize out = 9.7 - 5.4 = 4.3g
(c)(i) (I) Al\(_2\)O\(_{3(s)}\) + 6HCl\(_{(aq)}\) \(\to\) 2AlCl\(_{3(s)}\) + 3H\(_2\)O\(_{(l)}\)
(II) Al\(_2\)O\(_{3(g)}\) 2NaOH\(_{(aq)}\) + 3H\(_2\)O\(_{(l)}\) \(\to\) 2NaAl(OH)\(_4\)
(ii) Amphoteric
(iii) ZnO / PbO / SnO /Al\(_2\)O\(_3\)
d)(i) I [Al(H\(_2\)O)\(_6\)]\(^{3+}\) = x + (0) = +3
x = +3
Al = +3
(II) NaH = 1+ x = 0
x = —1
H = — 1
(ii)(I) Copper (II) tetraoxosulphate VI pentahydrate
(II) Calcium tripxocarbonate IV
(III) Potassium tetraoxomanganate VII.
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}