(a) Draw a labelled diagram of a vacuum flask. Explain how its construction minimizes heat exchange with the surroundings.
(b) State Boyle's law. A thread of mercury of length 15cm is used to trap some air in a capillary tube with uniform cross- sectional area and closed at one end with the tube vertical and the open end uppermost, the length of the trapped air column is 20cm. Calculate the length of the air column when the tube is held:
(i) Horizontally ; (ii) vertically with the open end underneath. [Atmospheric pressure = 76cm Hg]
(c) Explain why it is not advisable to sterilize a clinical thermometer in boiling water at normal atmospheric pressure.
(a) 
Vacuum between the double walls prevents heat loss by conduction and convection. The walls are silvered to prevent heat loss by radiation. The cover is made of poor conductor (cork) and it prevents evaporation.
(b) Boyle's law states that the volume of a given mass of gas is inversely proportional to its pressure provided its temperature is constant. Mathematically,
\(V = \frac{K}{P}\) where K is a constant of proportionality; P = Pressure ; V = Volume.
\(\therefore PV = K \implies P_{1} V_{1} = P_{2} V_{2}\)
(i) Atmospheric pressure (76cm Hg)
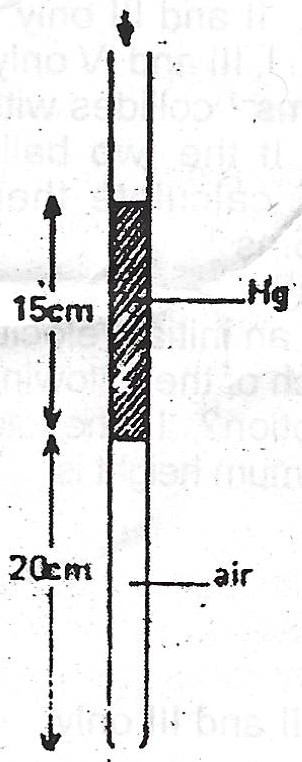
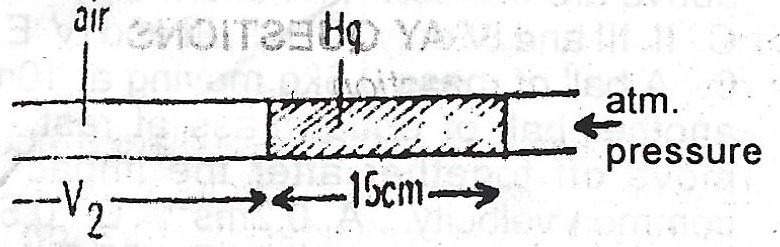
\(V_{1} = 20cm, V_{2} = ? , P_{1} = (15cm + 76cm) = 91 cmHg\)
\(P_{2} = 76 cmHg\)
Using Boyle's law,
\(P_{1} V_{1} = P_{2} V_{2}\)
\(V_{2} = \frac{P_{1} V_{1}}{P_{2}}\)
\(V_{2} = \frac{91 \times 20}{76} = \frac{1820}{76}\)
= \(23.95 cm \approxeq 24cm\)
Note: We have assumed that volume is proportional to length. Also, \(P_{2} = 76 cmHg\) because only the atmospheric pressure is acting in this case.
(ii) 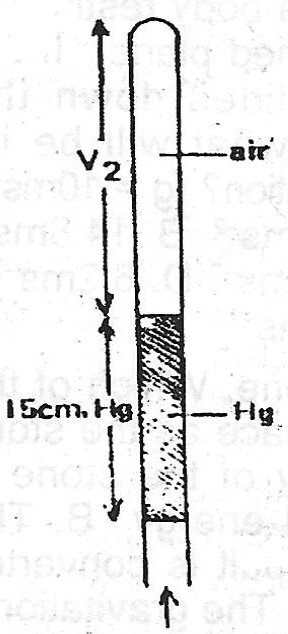
In this case, \(V_{1} = 20cm\), \(P_{1} = 91 cmHg \) as above ; In this case, \(V_{2} = ?\) but \(P_{2} = (76 - 15) cmHg = 61 cmHg\)
\(V_{2} = \frac{91 \times 20}{61} = \frac{1820}{61}\)
= \(29.84 cmHg \approxeq 30 cmHg\)

Clinical thermometer is used to determine the temperature of human body which is about 37°C for a healthy person. It is not advisable to sterilize a clinical thermometer in boiling water at normal atmospheric pressure because
(1) The range of the clinical thermometer is usually between 40°C and 50°C. Hence when put in boiling water it over expands since the temperature of boiling water is 100°C.
(2) The boiling water temperature will be too high for the glass material thus cracking it.
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}