(a) Using the kinetic theory of matter, explain why;
(i) Evaporation causes cooling
(ii) Boiling water changes to steam without any change in temperature, although heat is being supplied to the water.
(b) (i) State Boyle's law.
(ii) With the aid of a labelled diagram, describe an experiment to illustrate the relationship between the volume and pressure of a given mass of gas at constant temperature. (iii) State two precautions necessary to obtain accurate results.
(a)(i) Liquids consist of molecules in random motion having different velocities, hence near the surface molecules with higher velocities and kinetic energy escape from the surface. Therefore the average kinetic energy of the remaining liquid is reduced. Since the average kinetic energy is a measure of temperature, therefore temperature is lowered. Hence evaporation causes cooling.
(ii) The heat supplied to boiling water increases the kinetic energy of the molecules and also weaken the bonds between the molecules. Hence at boiling the heat supplied is used in breaking the bonds, therefore molecules escaped as steam without change in temperature.
(b) (i) Boyle's law states that the volume of a fixed mass of gas at constant temperature varies inversely as the pressure upon it.
(ii)
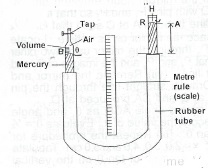
Introduce dry air into burette (B) and close the tap. Read and record the barometer reading H i.e. a.p., keeping B steady, raise the reservoir R, read the mercury head h from the scale, at the same time the corresponding volume V of air from the Repeat burette. Repeat the experiment with various values of H, and determine the corresponding values of h and v. Evaluate P = H ± h and V. Graph of P against V\(^{-1}\)
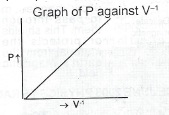
The graph P against V\(^{-1}\) is a straight line graph passing through the origin, showing that pressure is inversely proportional to volume.
(iii) Precautions;
– I ensured that dry air is used.
– I avoided parallax error when reading metre rule.
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}