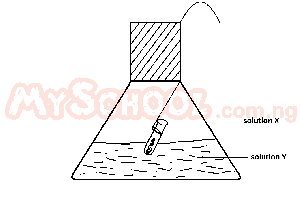
An experiment test of the Law of conservation of Mass is illustrated in the diagram. In practice, the flask is weighed before and after reaction between solutions X and Y. Which of the following pairs of solutions will be unsuitable for the experiments
Option E won't be suitable because it contains HCl (acid) and Sodium carbonate ( Carbonate) which will liberate CO\(_2\). Hence the mass of the product will not be same as the mass before the reaction.
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}