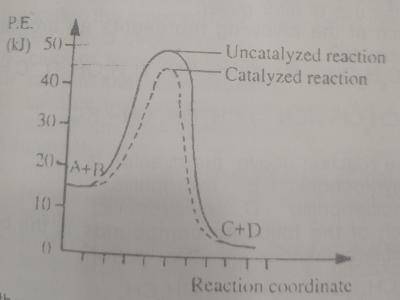
In the diagram above, the respective activation energies for the catalyzed and uncatalyzed reactions in kJ are
The activation energy, Ea is the energy of the transition state, (T.E) minus the reactant energy (R.E).
For Catalyzed reaction, Ea = T.E - R.E = 45 - 15 = 30
For Uncatalyzed reaction, Ea = T.E - R.E = 50 - 15 = 35
There is an explanation video available below.
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}