(a) Give the products of the following reactions:
(i) hydrolysis of simple proteins.
(ii) alkaline hydrolysis of fats and oils.
(b) A combustion tube was packed with small pieces of broken clay pot and the tube maintained at a temperature of 750K. When the vapour of decane was passed into the tube, the main products included a gaseous hydrocarbon X.
(i) Name the process involved in the reaction. Give its industrial application.
(ii) State the function of the pieces of broken pot in the experiment.
(iii) Give one chemical test to distinguish between X and methane.
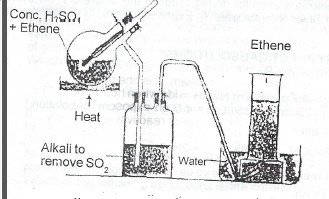
(iv) Draw a labelled diagram for the laboratory preparation of X.
(c)(i) State what would be observed if a piece of sodium was added to 10cm\(^3\) of propanol in a beaker. Write an equation for the reaction.
(ii) Give the main product formed when excess acidified potassium heptaoxodichromate (VI) reacts with each of the following: propan-1- ol: propan - 2 -of; State the type of process involved in the reactions.
(a)(i) Amino acids
(ii) Soap + glycerol
(b)(i) Cracking process. Conversion of the less demanded fractions to the more demanded in oil industry.
(ii) The pieces of broken pot is to avoid local heating and therefore create medium of uniform heating.
(iii) X is an alkene and will decolourize the reddish-brown colour of bromine vapour which methane cannot do.
(v) Laboratory preparation of ethene.
(c)(i) Effervescence occurs and hydrogen gas is evolved. 2CH\(_3\)CH\(_2\)CH\(_2\)OH + 2Na\(_{(S)}\) \(\to\) H\(_{2(s)}\) + 2CH\(_3\)CH\(_2\)CH\(_2\)ONa
(ii) Propan-1-ol and propan-2-one respectively. The process involved in the reaction is oxidation of alkanols.
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}