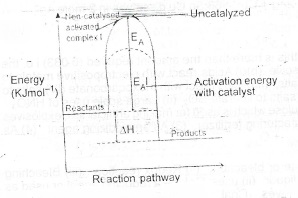
(a) Draw an energy profile diagram to illustrate a catalysed exothermic reaction and label parts of the curves representing the following:
(i) activated complex (without catalyst);
(ii) activated energy (with catalyst)
(iii) enthalpy change
(b) Give the reasons for the following observations:
(i) A balloon filled with liyilrogen becomes deflated faster than a balloon filled with air under the same conditions.
(ii) Hydrogen peroxide decomposes slowly at room temperature but when a pinch of MnO, is added, bubbles form rapidly.
(iii) A solution of hydrogen chloride as in methylbenzene has no effect on `litmus but a solution of the gas in water turns blue litmus paper red.
(c) Consider the reaction represented by the following equation: 2MnO\(^-_{4(aq)}\) + 5C\(_2\)O\(^{2-}_4\) + 16H\(^+\) \(\to\) 2Mn\(^{2+}_{(aq)}\) + 8H\(_2\)O\(_{(l)}\) + 10C\(_{2(g)}\) .
Write down: (i) the species undergoing reduction giving reasons;
(ii) the reducing agent giving reasons;
(iii) the reduction half equation;
(iv) one observation made during the reaction.
(d)(i) What is an electrochemical cell?
(ii) State three differences between an electrochemical cell and an electrolytic cell.
(a)
(b)(i) Hydrogen is lighter or-4ess dense than air. The hydrogen particles therefore diffuse faster than air.
(ii) The MnO\(_2\) catalyses the decomposition of the hydrogen peroxide into-water and oxygen
(iii) HCl is covalent, it produces no ions in a non-polar solvent like methylbenzene. In water it ionizes to give H\(^+\) or H\(_{3}\)O\)(^+\) as the only positive charge which turns blue litmus paper red.
(c)(i) MnO\(^{-4}\). Since oxidation number of Mn decreases from +7 to +2.
(ii) C\(_2\)O\(_4\)\(^{-2}\). Since the oxidation number of carbon increases from +3 to +4
(iii) MnO\(^{-4}\) + 8H\(^+\)+ 5e\(^-\) \(\to\) Mn\(^{2+}\) + 4H\(_2\)O
(d)(i) An electrochemical cell is a device which converts chemical energy into electrical energy.
ii)
|
Electrochemical cell |
Electrolytic cell |
|
1. Chemical reaction produces electricity |
Electric current is required for the reaction to occur |
|
2. Electrons are produced as a result of oxidation at an electrode |
Electrons are pushed by an outside source such as a battery. |
|
3: Cathode is the positive electrode |
Cathode is the negative electrode |
|
4. Anode is negative electrode |
Anode is positive electrode |
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}