(a)(i) Draw the energy profile diagram for the reaction; H\(_{2(g)}\) + I\(_{2(g)}\) \(\to\) 2Hl\(_{(g)}\); \(\Delta\)H = –13 KJ mol\(^{-1}\)
(ii) If the concentration of HI\(_{(g)}\) increases from 0.000 to 0.002 mol dm\(^{-3}\) in 80 seconds, what is the rate of t reaction?
(b)(i)Give one use of each of the following compounds: I. NaHCO\(_{3}\); II. CaSO\(_{4}\); III. CaCO\(_{3}\).
(ii) State a drying agent that can be used for each of the following gases: I. SO\(_{2}\); II. HCI; Ill. NH\(_{3}\)
(c)(i) Write an equation for the complete combustion of carbon in oxygen.
(ii) Calculate the number moles of carbon (IV) oxide produced from the complete combustion of 2.5 g of carbon. [ C = 12.0, O = 16]
(iii) Mention one use of I. carbon (II) oxide; II. carbon (IV) oxide.
(d) An industrial raw material has the following composition by mass:
Iron = 28.1%; Chlorine = 35.7%; Water cf crystallization = 36.2%.
Calculate the formula for the material. [H = 1.00, O = 16.0, CI = 35.5, Fe = 56.0]
(e) Give one example of a (i) metal that is liquid at room temperature,
(ii) non-metal that is liquid room temperature.
(a)(i)
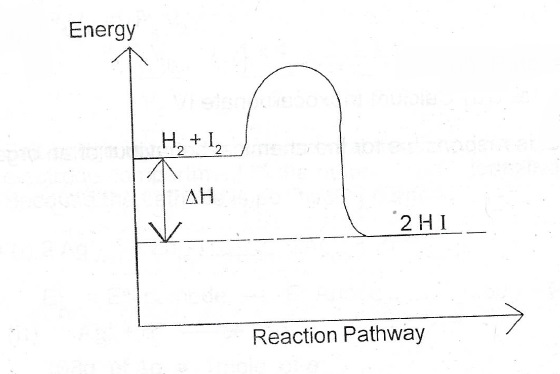
(ii) Rate = change in concentration = \(\frac{0.002 — 0.000}{80}\)
= \(\frac{0.002}{80}\)
= 2.5 x 10\(^{-5}\) moldm\(^{-3}\) sec\(^{-1}\)
(b) (i) (I) Manufactise of baking powder (II) Manufacture of tetraoxosulphate (VI) plaster of paris (P.O.P.)
(III) Cement making, extraction of iron, solvay process, manufacture of glass, manufacture of putty
(ii) (I) Conc. H\(_2\)SO\(_4\)
(II) Conc. H\(_2\)SO\(_4\)
(III) CaO / Silica gel.
(c)(i) C\(_{(s)}\) + O\(_{2(g)}\) \(\to\) CO\(_{2(g)}\)
(II) 12g C gives 1 mol CO\(_2\)
2. 5g C gives \(\frac{1 \times 2.5}{12}\)
= 0.208 mol.
(iii)(I) Extraction of metals, manufacturer of fuel, as gaseous fuel. (II) * Manufacture of fizzy drinks * Fire extinguisher * Cooling nuclear reactors *Refrigeration.
(d) Mole: Fe = \(\frac{28.1}{56}\); Cl = \(\frac{35.7}{35.5}\); H\(_2\)O = \(\frac{36.2}{18}\)
Mole ratio; Fe = \(\frac{0.5}{0.5}\); Cl = \(\frac{1}{0.5}\); H\(_2\)O = \(\frac{2}{0.5}\)
Fe =1; Cl = 2; H\(_2\)O = 4
(e)(i) Mercury (H\(_g\)) (ii) Bromine (Br\(_2\))
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}