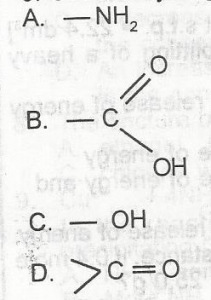
Which of the following functional groups will give gas bubbles when treated with a saturated solution of sodium hydrogen trioxocarbonate (IV)?
When a carboxylic acid (RCOOH) reacts with sodium bicarbonate (NaHCO\(_3\)), it forms a salt (RCOONa), water (H\(_2\)O), and carbon dioxide (CO\(_2\)). The carbon dioxide gas produced in this reaction is what causes the bubbles or effervescence observed.
This reaction is a simple and reliable test to distinguish carboxylic acids from other organic compounds, as most other functional groups (like alcohols or phenols) are not acidic enough to react with bicarbonate and produce a gas.
There is an explanation video available below.
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}