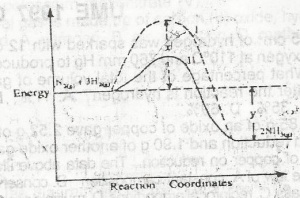
Use the diagram above to answer this question. The activation energy of the uncatalysed reaction is
Activation energy is the minimum amount of energy required for a chemical reaction to occur. Graphically, it is the difference between the peak of the hump and the reactant energy.
Consequently, the correct answer is option A.
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}