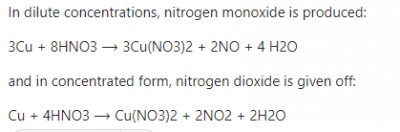
Generally, when metals react with acids, Hydrogen gas is usually liberated, with an exception of dilute Trioxonitrate(V) acids. However, in this case, it is not the usual Acid- metal reaction, as Copper metal is reacting with concentrated nitric acid. Thus, Copper is oxidized by the concentrated nitric acid, HNO3, to produce Cu2+ ions while the nitric acid is reduced to nitrogen dioxide, NO2 and water is also formed. Therefore, the correct answer is option D.
Contributions ({{ comment_count }})
Please wait...
Modal title
Report
Block User
{{ feedback_modal_data.title }}